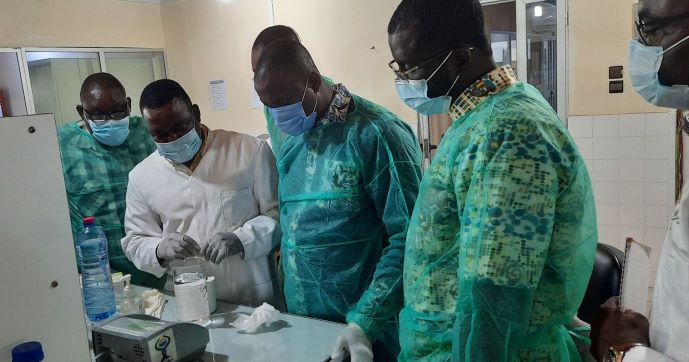
Malaria is the leading cause of mortality among children under five and the leading cause of morbidity among adults in Benin, according to the U.S. President’s Malaria Initiative (PMI). Along with its partners, PMI supports interventions that include large-scale implementation of artemisinin-based combination therapy (ACT) and intermittent preventive treatment for pregnant women. However, the spread of substandard and falsified ACTs could reverse progress and promote the continued emergence and spread of antimicrobial resistance.
PQM+ is helping the country’s national quality control laboratory (l'Agence Nationale de Contrôle de la Qualité des Produits de Santé et de l'Eau, ANCQ) to strengthen its quality management system to achieve international accreditation, such as ISO/IEC 17025 or WHO prequalification. Aside from the ANCQ, PQM+ is also working with the National Metrology Laboratory (Agence Nationale de Normalisation, de Métrologie et du Contrôle de la Qualité, ANM) to expand their ISO/IEC 17025 accreditation for temperature and pressure. PQM+ is also working with Benin’s medical products regulatory body, the Beninois Agency for Medicines and other health products (Agence Béninoise du Médicament et des autres produits de Santé (ABMed), to strengthen its national post-marketing surveillance systems to help ensure the quality of medical products on the local market. Furthermore, PQM+ is supporting ABMed to improve its system for regulatory inspections by adopting a risk-based inspection approach to strengthen their regulatory inspection function.