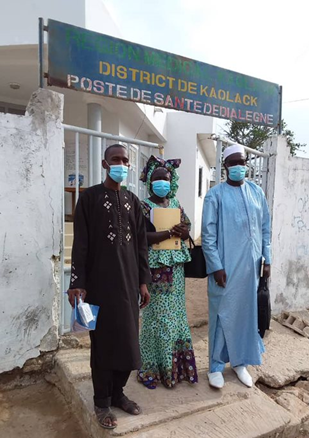
The Government of Senegal seeks to build an efficient system of regulation and control to ensure the quality of medicines in the country. PQM+ is supporting the Senegalese Pharmaceutical Regulatory Agency (l’Agence Sénégalaise de Réglementation Pharmaceutique, (ARP)) to attain WHO GBT Maturity Level 3 and is also working with ARP’s quality control laboratory (DICQ) to achieve international accreditation, such as ISO/IEC 17025 or WHO prequalification. The program is supporting Senegal to conduct risk-based post-marketing surveillance (RB-PMS) of antimalarials to gather data that will inform regulatory decisions around substandard and falsified medical products in the country. PQM+ is also supporting the government’s response to the COVID-19 pandemic by collaborating with ARP to build its capacity to regulate vaccines and other biologicals to be produced locally.